Section 7: Strategies for management of osteoporosis and fracture risk
Duration and monitoring of bisphosphonate treatment
Osteoporosis is a long-term condition for which there is currently no cure, therefore life-long treatment and monitoring to prevent fractures is often required.
Recommendations
- Plan to prescribe oral bisphosphonates (alendronate, ibandronate and
risedronate) for at least 5 years and then re-assess fracture risk. Longer durations of treatment, for at
least 10 years, are recommended in the following men and women (Strong recommendation) (see Figure 4):
- Age ≥70 years at the time that the bisphosphonate is started
- Who have a previous history of a hip or vertebral fracture(s)
- Treated with oral glucocorticoids ≥7.5 mg prednisolone/day or equivalent
- Who experience one or more fragility fractures during the first 5 years of treatment (if treatment is not changed).
- Plan to prescribe intravenous bisphosphonate (i.e., zoledronate) for at
least 3 years and then re-assess fracture risk. Longer durations of treatment, for at least 6 years, are
recommended in the following men and women (Strong recommendation) (see Figure
5
):
- Age ≥70 years at the time that the bisphosphonate is started
- Who have a previous history of a hip or vertebral fracture(s)
- Treated with oral glucocorticoids ≥7.5 mg prednisolone/day or equivalent
- Who experience one or more fragility fractures during the first 3 years of treatment (if treatment is not changed).
- If a new fracture occurs after bisphosphonate treatment is discontinued, reassess using FRAX and restart treatment (Strong recommendation).
- If bisphosphonate treatment is discontinued and no new fracture occurs, reassess using FRAX after 18 months for risedronate and ibandronate, 2 years for alendronate, and 3 years for zoledronate to inform whether treatment should be restarted (Strong recommendation).
Evidence Summary
- Bisphosphonate therapy is associated with rare but serious adverse events, notably atypical femoral fractures (AFFs) and osteonecrosis of the jaw (ONJ). Defining optimal duration of bisphosphonate therapy attempts to ensure that the benefit in fracture risk reduction outweighs the small risk of AFFs and ONJ at all time points through patient management.
- Bisphosphonates are retained long term in bone allowing the beneficial effects to persist for some time after cessation of treatment administration. This has raised the possibility that some patients may benefit from a period off treatment to restore the benefit/risk balance 256; (Evidence level IIa), in which treatment is stopped after some years and the need for reinstitution of therapy is subsequently reassessed. Treatment review in patients taking bisphosphonates is therefore critical 257 and each patient must be assessed individually to assess relative risks and benefits; there is no standard policy for ‘all patients’ 214; (Evidence level IIa). Because pivotal clinical trials have mostly been limited to a duration of three years, recommendations for longer term use and for pauses in treatment are based on limited evidence from extension studies in postmenopausal women 258,259;(Evidence level IIa). There is currently no evidence on which to base specific recommendations for men.
- Withdrawal of bisphosphonate treatment is associated with decreases in BMD and increased bone turnover after 2-3 years for alendronate260,261;(Evidence level Ib), and 1-2 years for ibandronate and risedronate262,263;(Evidence level Ib). In the case of zoledronate, withdrawal after 3 years’ treatment is associated with only a small decrease in BMD after a further 3 years without treatment264; (Evidence level Ib). Comparison between offset of alendronate and zoledronate at 3 years showed alendronate-treated patients had greater reductions in total hip BMD and greater rises in PINP, despite a longer treatment exposure with alendronate, supporting a more rapid offset of drug effect than with zoledronate265; (Evidence level IIb).
- In the Fracture Intervention Trial Long-term extension study of alendronate (FLEX), there were significantly fewer clinical vertebral fractures in women previously treated with alendronate for 5 years who continued with alendronate for five more years than in those assigned to placebo after 5 years of alendronate261; (Evidence level Ib). In the Health Outcomes and Reduced Incidence with Zoledronate Once Yearly (HORIZON) study extension, the risk of morphometric vertebral fractures was significantly lower in women continuing on zoledronate for 3 years after the initial three years therapy when compared to those switched to placebo264; (Evidence level Ib). Post-hoc analyses from the alendronate and zoledronate extension studies suggest that women most likely to benefit from long-term bisphosphonate therapy are those with low hip BMD (T-score 70;(Evidence level Ib). Older age was also associated with increased fracture risk after discontinuation of alendronate therapy266;(Evidence level Ib).
Reassessment of fracture risk in individuals on osteoporosis drug treatment
Recommendations
- Review treatment adherence in men and women who sustain a fragility fracture whilst on drug treatment, (poor adherence is when less than 80% of treatment has been taken correctly) and investigate for secondary causes of osteoporosis (Strong recommendation).
- Fracture risk assessment in patients receiving drug treatment should be performed using FRAX with BMD, with arithmetic adjustments to FRAX probabilities to take account of additional clinical risk factors ( see Section 3). If the FRAX-derived fracture probability exceeds the intervention threshold drug treatment should be continued (Strong recommendation).
- If biochemical markers of bone turnover indicate relapse from suppressed bone turnover and/or BMD has decreased following bisphosphonate withdrawal, consider resumption of drug treatment (Conditional recommendation).
- After 10 years of bisphosphonate treatment, patient management should be considered on an individual basis (Conditional recommendation).
Evidence Summary
- Stopping osteoporosis treatment, be it with bisphosphonate or denosumab, is associated with an increased risk of fragility fracture, such that routine cessation of anti-resorptive therapy (so called ‘drug holidays’) is not supported by review of the evidence 214; (Evidence level IIa).
- Reassessment of fracture risk in treated individuals can be performed using FRAX with femoral neck BMD 149; (Evidence level IIb). The NOGG intervention thresholds can then be used to guide the decision as to whether treatment can be stopped for a period of time (Figures 4 and 5). Whereas FRAX cannot be used to assess treatment response149; (Evidence level IIb) it does have a role in reassessing current fracture risk to determine the need to continue or to discontinue treatment.
- Detection of the offset of drug effect, using BMD and bone
turnover changes, potentially provides information to influence clinical management. However, there are
presently no definitive data that link a potential threshold change in BMD or bone turnover markers during
drug offset to clinically meaningful changes in fracture risk.
Figure 4: Oral Bisphosphonates: Clinical Flowchart for long term treatment and monitoring
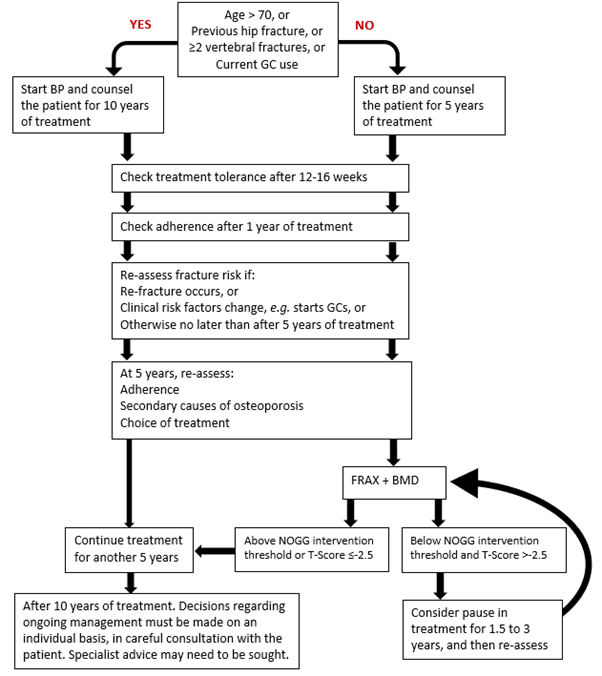
GC: Glucocorticoids (oral ≥7.5 mg prednisolone/day or equivalent).
BP: bisphosphonateFigure 5: Intravenous Bisphosphonates: Clinical Flowchart for long term treatment and monitoring
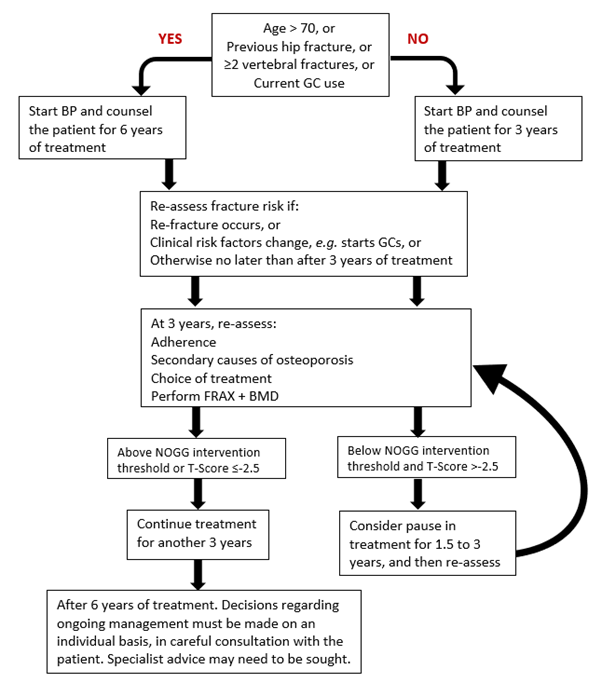
GC: Glucocorticoids (oral ≥7.5 mg prednisolone/day or equivalent).
BP: bisphosphonate
Rare adverse effects of long-term bisphosphonate and denosumab treatment
Recommendations
- During bisphosphonate or denosumab therapy, encourage all patients to maintain good oral hygiene, receive routine dental check-ups, and report any oral symptoms such as dental mobility, pain, or swelling (Strong recommendation).
- In those with severe dental disease who require bisphosphonate or denosumab treatment, timely dental review and dental treatment by an appropriately experienced dental surgeon should be pursued before drug administration, bearing in mind drug treatment should be initiated as soon as possible after a fragility fracture; a multi-disciplinary team (MDT) approach to discuss individual needs is encouraged (Conditional recommendation).
- During bisphosphonate or denosumab treatment, although ideally patients should minimise invasive dental procedures where possible, if indicated they can be carried out safely and successfully in most patients. When dental procedures are required, there are no data available to show whether treatment discontinuation reduces the risk of ONJ. Clinical judgment of the treating physician should guide the management plan of each patient based on individual benefit/risk assessment, ensuring patients continue to access routine dental care (Conditional recommendation).
- During bisphosphonate or denosumab therapy, advise patients to report any unexplained thigh, groin or hip pain and if such symptoms develop, the femur should be imaged (by full length femur X-ray, isotope scanning or MRI) (Strong recommendation).
- If an AFF is identified, image the contralateral femur (Strong recommendation).
- All patients who develop an AFF should be referred to an osteoporosis specialist to guide management of future bone health (Strong recommendation).
- In patients who develop an AFF, discontinue bisphosphonate or denosumab treatment (Conditional recommendation).
Evidence Summary
- Osteonecrosis of the jaw (ONJ) occurs only very rarely in patients receiving bisphosphonate or denosumab therapy for osteoporosis. The estimated incidence in those receiving bisphosphonates is 10-100/100,000 person-years of exposure in clinical trials. Risk factors for ONJ include poor oral hygiene, dental disease, dental interventions, smoking, cancer, chemotherapy and/or glucocorticoid therapy 267, 268; (Evidence level IIa). The incidence of ONJ is substantially greater with the higher doses of bisphosphonates or denosumab that are used to treat patients with skeletal metastases. The Scottish Dental Clinical Effectiveness Programme has produced guidance on oral health management in patients taking anti-resorptive medication 269.
- Osteonecrosis of the external auditory canal after bisphosphonate treatment has been described very rarely in case reports, with patients presenting with ear symptoms including chronic ear infections. Possible risk factors include steroid use and chemotherapy and/ or local risk factors such as infection or trauma. 270; (Evidence level IV).
- Atypical femoral fractures (AFF), mainly of the subtrochanteric and diaphyseal regions of the femoral shaft, have been reported rarely in patients taking bisphosphonates or denosumab for osteoporosis. Asian race, femoral bowing and glucocorticoid use have been identified as risk factors 271. In a recent review by the ASBMR Task Force on the management of osteoporosis in patients on long-term bisphosphonates, a systematic search of the literature revealed that the absolute risk was consistently low, ranging between 3.2-50 cases/100,000 person-years of exposure 272, 273; (Evidence level IV). This estimate appeared to double with prolonged duration of BP use (> 3 years, median duration 7 years), and declined with discontinuation 272, 273; (Evidence level IV), 274; (Evidence level IIa).
- In a nationwide cohort study from Denmark, use of alendronate in excess of 10 years was associated with a 30% lower risk of hip fracture and no increase in the risk of fractures of the subtrochanteric femur and femoral shaft, supporting an acceptable risk benefit balance in terms of fracture outcomes 275; (Evidence level IIb).
- Atypical femoral fractures are often bilateral, associated with prodromal pain and tend to heal poorly. Prodromal pain can be felt in the thigh, groin or hip for days, weeks or months before fracture. Discontinuation of bisphosphonate or denosumab therapy is advised in patients who develop an atypical fracture, weight-bearing activity should be restricted, adequate calcium and vitamin D should be ensured, and alternative treatment options considered where appropriate. Surgical treatment with intramedullary nailing is often recommended 272 ,273; (Evidence level IV).
- There is a lack of good quality evidence on the medical management of bone health following an AFF. However, a recent international expert consensus document supported by a systematic review proposed practical measures to help in patient management 276; (Evidence level IV). Following an AFF, if risk of fragility fracture is low, further pharmacological bone treatments can be avoided. If fracture risk is high and bilateral surgical fixation of fractures has been performed, consider use of teriparatide. If unilateral or no surgical intervention has taken place, consider teriparatide, romosozumab, raloxifene, or HRT. The potential utility of teriparatide as an adjunct to healing following AFF has been examined. There is no evidence that teriparatide enhances healing of AFFs, but limited data show a tendency towards faster healing in surgically managed AFFs (complete and incomplete). However, in AFFs managed conservatively, there was no suggestion of improved fracture healing with teriparatide 276; (Evidence level IV). The benefits versus risks of using bisphosphonates or denosumab after AFF should be carefully examined if these options are considered, taking into consideration prior unilateral or bilateral nailing, use of an anabolic agent post AFF, together with the overall clinical situation and fracture risk (Evidence level IV).
Glucocorticoid-induced osteoporosis
Recommendations
- Because bone loss and increased fracture risk occur early after
initiation of oral glucocorticoids, bone-protective treatment should be started in the following people,
at the same time as glucocorticoid therapy without waiting for bone density assessment, which should
follow later (Strong recommendations):
- anyone with a prior fragility fracture,
- women age ≥70 years,
- postmenopausal women, and men age ≥50 years, prescribed high doses of glucocorticoids, i.e., ≥7.5 mg/day of prednisolone or equivalent over 3 months (N.B., this is equivalent to ≥30mg/day of prednisone for 4 weeks over 3 months)
- postmenopausal women, and men age ≥50 years, with a FRAX probability of major osteoporotic fracture or of hip fracture exceeding the intervention threshold.
- Oral bisphosphonates (alendronate or risedronate) or intravenous zoledronate are the most cost-effective first-line drug options for bone protection. Denosumab is an alternative option. Teriparatide can be a first-line drug option in those at very high fracture risk (Strong recommendation).
- Adequate calcium intake should be achieved through dietary intake if possible, with the use of supplements if necessary. An adequate vitamin D status should be maintained, using supplements if required (Strong recommendation).
- If glucocorticoid therapy is stopped, withdrawal of bone-protective therapy may be considered at the same time, provided on re-assessment of fracture risk using FRAX, the probabilities of both major osteoporotic fracture and of hip fracture lie below the intervention threshold (Strong recommendation).
- If glucocorticoids are continued long term, bone protection should be maintained in the majority of cases (Strong recommendation).
- Patients starting medium or low dose oral glucocorticoid therapy who have a FRAX probability near to, but below the intervention threshold, should have FRAX with BMD reassessed 12-18 months after starting glucocorticoid therapy (Conditional recommendation).
Abaloparatide and romosozumab are further options for treatment if their therapeutic indication is fulfilled, i.e., in postmenopausal women at very high fracture risk.
Bone protective therapy may be appropriate in some premenopausal women and younger men, particularly in individuals with a previous history of fracture, or those receiving high doses of glucocorticoids (≥7.5 mg/day of prednisolone or equivalent over 3 months). Caution is advised when prescribing drug treatment in women of childbearing age. Referral of complex cases to secondary care is often necessary.
Evidence Summary
- Although guidance on the prevention and management of glucocorticoid-induced osteoporosis has been developed in many countries, there is evidence that in the UK osteoporosis risk assessment and management are still inadequate in long-term users of oral glucocorticoids 277; (Evidence level IIIb). Bone loss and increased fracture risk occur rapidly after initiation of oral glucocorticoid therapy and increase with the dose of glucocorticoids 55, 278. The increase in fracture risk is seen for vertebral and non-vertebral fractures, including hip fractures, and is partially independent of BMD 56; (Evidence level Ia).
-
Approval for the use of bone protective therapy to prevent osteoporosis in people receiving oral glucocorticoids was based mainly on BMD bridging studies carried out as part of Phase III randomised controlled trials with bisphosphonates190,195,202,279,280. Subsequently approval has been given for denosumab using the same methodology 205. Fracture prevention has not been considered as an efficacy end-point in most trials. However, although not a primary end-point, in an 18-month randomised controlled trial extended to 36-months comparing teriparatide with alendronate significantly fewer subjects in the teriparatide group had vertebral fractures compared with the alendronate arm 243, but with no benefit on non-vertebral fractures. This protection against vertebral fractures was confirmed in a recent meta-analysis which showed that co-prescription of teriparatide, alendronate, risedronate or denosumab with glucocorticoids could reduce the incidence of vertebral fractures, with further evidence of a reduction in non-vertebral fracture rates with alendronate or teriparatide (Table 7) 281;(Evidence levels Ia & Ib).
Table 7: Effect of approved interventions for glucocorticoid-induced osteoporosis on BMD and fracture risk.NAE: No available evidence. Bone protective therapy Spine BMD Hip BMD Vertebral fracture Non-vertebral fracture Evidence of superiority for spine and/or hip BMD Alendronate Ib Ia Ia Ia Inferior to teriparatide (Ib) Risedronate Ib Ia Ia NAE Inferior to zoledronate (Ia) Zoledronate Ib Ib Ia NAE Superior to risedronate (Ib) Denosumab Ib Ia Ia NAE Superior to bisphosphonates (IIa) Teriparatide Ib Ib Ia Ia Superior to alendronate (Ib) - Considering the increased fracture risk associated with higher glucocorticoid doses, FRAX assessment provides fracture probabilities based on both an average dose of prednisolone (2.5–7.5 mg/day or its equivalent), and a higher dose (≥7.5 mg/day or its equivalent). Individuals taking an average dose of prednisolone 85; (Evidence level IIb). For very high doses of glucocorticoids, i.e., >20mg/day prednisolone or its equivalent, greater upward adjustment of fracture probability is required 55; (Evidence level IIa).
-
When the UK FRAX model is used and the glucocorticoid box is filled, 2 points appear on the NOGG graphs, one for medium dose and one for high dose (all defined as above). The assessment thresholds (fracture probabilities for BMD testing) and intervention thresholds (fracture probabilities for therapeutic intervention) are then used in the same way as described for postmenopausal women and older men.
Table 8: Adjustment of FRAX derived fracture probability estimates according to daily dose of prednisoloneDose Prednisolone equivalent dose (mg/day) Average adjustment to hip fracture probability Average adjustment to major osteoporotic fracture (MOF) probability Low -35% -20% Medium 2.5-7.5 None None High ≥7.5 +20% +15%
Men receiving androgen-deprivation therapy
Recommendations
The NOGG supports the recent guideline published by Brown et al 2020 282.
- All men starting androgen deprivation therapy (ADT) should have their fracture risk assessed using FRAX, considering ADT use as a secondary cause of osteoporosis, with BMD measured where available (Strong recommendation).
- Consider referring men, with high fracture risk requiring drug treatment, to secondary care for assessment and initiation of treatment with bisphosphonates or denosumab (Conditional recommendation).
- Men with FRAX probability near to, but below the intervention threshold, and patients going on to additional systemic therapies (particularly those requiring glucocorticoids), should have FRAX with BMD reassessed 12-18 months after starting ADT (Conditional recommendation).
Evidence Summary
- There is no evidence that skeletal metabolism in men differs fundamentally from that of women 283. However, secondary causes of osteoporosis are common in men and amongst these hypogonadism is prominent 284. Androgen deprivation therapy (ADT), used primarily in the treatment of older men with prostate cancer, is frequently associated with hypogonadism. Osteoporosis caused by ADT is associated with rapid loss of BMD within 6–12 months of initiation of ADT 285; (Evidence level Ic). There is a significant increase in fracture risk in men with prostate cancer in the 5 years following the initiation of ADT when compared to those not receiving ADT 286; (Evidence level Ic).
- Bisphosphonates and denosumab are effective drug treatments for preventing BMD loss in men with prostate cancer taking ADT, although effects on fracture risk have not been demonstrated. Exercise programmes are a less effective alternative which are insufficient in isolation287; (Evidence level Ib).
- In a systematic review and network meta-analysis all evaluated treatments for ADT-induced bone loss, which included bisphosphonates and selective oestrogen receptor modulators (SERMs), were effective in improving BMD compared to placebo. However, zoledronate generated greater improvements in BMD compared to other drug treatments at all bone density sites, except for risedronate which had better BMD improvement compared to zoledronate at the femoral neck site in one small study 288; (Evidence level IIa).
- d. A recent UK consensus statement on prostate cancer treatment-induced bone loss concluded that fracture risk should be calculated using FRAX, considering ADT use as a secondary cause of osteoporosis, and including BMD where available and practical. BMD should always be assessed where calculated fracture risk is close to the NOGG intervention threshold. Those with FRAX probability near to, but below the intervention threshold, and patients going on to additional systemic therapies, should have FRAX with BMD repeated after 12-18 months 282; (Evidence level IIa).
Women receiving aromatase inhibitor therapy
Recommendations
- All women starting aromatase inhibitor (AI) therapy should have their fracture risk assessed using FRAX, considering AI use as a secondary cause of osteoporosis, including BMD measurement where practical (Strong recommendation).
- Women with high fracture risk should be commenced on drug treatment to prevent osteoporosis and fracture, with bisphosphonates or denosumab (Strong recommendation).
- Women with a FRAX probability near to, but below the intervention threshold, and patients going on to additional systemic therapies (particularly those requiring glucocorticoids), should have FRAX with BMD reassessed 12-24 months after starting AI therapy (Conditional recommendation).
- If adjuvant high-dose bisphosphonate therapy is used as part of breast cancer management, consider assessing fracture risk at the end of this bisphosphonate therapy, particularly if AI therapy continues (Conditional recommendation).
Evidence Summary
- The use of aromatase inhibitors (AI) in postmenopausal women induces bone loss at an average rate of 1-3% per year at sites rich in trabecular bone. Bone loss is more marked in young women with treatment-induced ovarian suppression, losing an average of 7-8% per annum 287; (Evidence level IIa).
- In case-control studies the incidence of fracture in women with breast cancer treated with AI is reported to be around 18-20% after 5 years follow-up 288. NICE guidance on management of early breast cancer, which recognises the excess risk of osteoporosis with the use of AIs, recommends a baseline DXA scan to assess BMD at the time of initiation of AI therapy 289;(Evidence level IV). International Consensus Position Statements suggest that fracture risk should be assessed, although the consideration of AI use as a secondary cause of osteoporosis in FRAX, may not adequately estimate fracture risk 288,290 ; (Evidence level IIa) with drug treatment to prevent bone loss and fractures recommended in those with a T-score of less than -2, or less than -1.5 with 1 additional risk factor, or in those with 2 or more risk factors (without BMD). Drug treatment should be a bisphosphonate (oral or parenteral) or denosumab, used in the doses as for postmenopausal osteoporosis. Denosumab and zoledronate both lead to significant gains in BMD at the spine and hip in postmenopausal women with breast cancer receiving AI, and both denosumab and risedronate have been shown to reduce fracture risk 291; (Evidence level Ia).